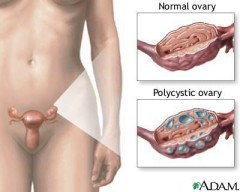
Polycystic ovarian syndrome (PCOS), also known as Stein-Leventhal syndrome, affects 6-10 % of women of reproductive age. Characteristic features of PCOS include menstrual cycle abnormalities and hyperandrogenism. Menstrual cycle abnormalities range from a decreased frequency of menses to complete amenorrhea, though some patients have normal menstrual cycles. Abnormal uterine bleeding can occur. Fertility is decreased. Manifestations of hyperandrogenism include acne, alopecia, and hirsutism. Recently hyperinsulinemia and insulin resistance have been recognized as features of PCOS (hyperandrogenism can lead to insulin resistance, and vice versa), and women with PCOS are at increased risk of type II diabetes mellitus. Obesity is common. The pathophysiology of PCOS is incompletely understood and the components of PCOS interact with each other in a complex manner. For example, obesity can lead to insulin resistance which can lead to hyperandrogenism.
Diagnosis of PCOS requires the exclusion of other causes of hyperandrogenism and anovulation/oligo-ovulation. Since pituitary or thyroid disease can cause ovulatory dysfunction, a prolactin level and TSH should be checked. Although the luteinizing hormone (LH)/ follicle stimulating hormone (FSH) ratio is usually greater than 2.5 to 3, a normal ratio does not exclude the diagnosis. A pregnancy test should also be checked. Androgen-producing neoplasms can be excluded by checking total testosterone and dehydroepiandrosterone sulfate (DHEAS) levels. Total testosterone levels are often mildly elevated in PCOS, but a level greater than 200 ng/dl suggests a virilizing neoplasm. 17-hydroxyprogesterone should be checked to screen for late-onset congenital adrenal hyperplasia. Sometimes a dexamethasone suppression test is performed to rule out Cushing’s syndrome. Non-obese patients should be screened for anorexia nervosa.
A transvaginal ultrasound is sometimes obtained in patients with PCOS; this test can identify most virilizing tumors. However, patients with PCOS do not always have radiographically demonstrated polycystic ovaries. In addition, approximately 25% of women with normal ovulation have polycystic-appearing ovaries.
Patients with PCOS are at increased risk for cardiovascular disease due to hyperandrogenism. Therefore fasting lipids should be checked. The patient should be assessed for other cardiac risk factors, such as smoking and hypertension. Due to the association between PCOS and insulin resistance, a fasting glucose level should be checked. Some also recommend checking insulin levels or glucose tolerance testing.
One of the primary treatments for PCOS is oral contraceptives, which suppress androgens. Sometimes spironolactone, which suppresses enzymes in the androgen biosynthetic pathway, is combined with oral contraceptives. Fertility can be increased by clomiphene citrate. Metformin, an insulin-sensitizing agent, has been shown to restore menstrual regularity. Weight loss is also helpful.
Several studies suggest that PCOS is more common in women with bipolar disorder or epilepsy than in the general population. Valproate probably increases the risk of PCOS. However, since the disorders that valproate is used to treat are also associated with PCOS, valproate has not been conclusively proven to be a causative factor for PCOS.
Psychiatrists should take a detailed menstrual history in female patients with bipolar disorder. It is also important to ask about hirsutism. Patients with baseline abnormalities should be referred to a primary care doctor for further evaluation. The development of PCOS symptoms during treatment also warrants referral to a primary care doctor. Symptoms of PCOS often remit or improve after the discontinuation of valproate. Prolactin-elevating antipsychotics can also cause menstrual abnormalities, and occasionally hirsutism.
--------------------------------------------------------------------
the above is for a psychiatry newsletter article I am writing